The disperse dyeing of cellulose acetate materials is a simple direct dyeing process. The dispersion of disperse dyes in warm water is sieved into the bath, possibly already containing additional dispersant. Boiling water and concentrated solutions of dispersing agents must be avoided as they can adversely affect the dye particle dispersion. Cellulose diacetate is dyed at temperatures not exceeding 85 °C, because of the risk of acetyl group hydrolysis on the fibre surface, which causes considerable dulling of the attractive lustre of the bright filaments. Because this thermoplastic material readily forms permanent creases at the usual dyeing temperature of 80–85 °C, dyeing of the full width fabric on a roller is necessary. A typical jig dyeing procedure of disperse dyeing involves two ends at 40–50 °C, followed by two ends at each higher bath temperature, up to the final dyeing temperature of 80–85 °C. At the higher temperatures, the lengthways tension must be as low as possible, to avoid elongation of the fabric. Beam dyeing is possible provided that the material allows good liquor flow through the roll at a pressure low enough to avoid deforming the plastic filaments.
Disperse dyes for cellulose acetate varies widely in their rates of exhaustion and levelling ability. Dyeing with mixtures of compatible dyes is essential. The SDC gives testing procedures for dyeing cellulose diacetate with disperse dyes. These tests establish the migration ability of the dye, the influence of temperature on dye uptake (temperature range test), the rate of dyeing and the colour build-up with increasing dye concentration relative to standard dyes of known properties.
The results of the temperature range test provide classic examples of the influence of temperature on dyeing kinetics and equilibrium. For dyes that adsorb rapidly at 50–60 °C, the amount of dye absorbed after dyeing for an hour will decrease as the dyeing temperature increases. This is the expected effect of temperature on an exothermic dyeing process that has reached or come close to equilibrium. The exhaustion (equilibrium constant) decreases with increasing temperature. For slow dyeing dyes, the amount of dye absorbed in one hour increases steadily with increasing dyeing temperature because this increases the rate of diffusion of dye into the fibre. After dyeing for one hour, the dyeing may be sufficiently far from equilibrium that the expected decrease of the exhaustion with increasing temperature does not occur. Some dyes may show a temperature of maximum dye exhaustion, showing the effects of temperature on dyeing rate at lower temperatures and on exhaustion at higher values. Slow dyeing dyes with poor temperature range properties will likely cause ending and listing when dyeing on a jig because the fabric ends and selvages tend to be cooler than the bulk of the material.
Blacks can be obtained in one of two ways. The simplest involves the use of a mixture of dull red, blue and yellow or orange disperse dyes at relatively high total concentrations. With appropriate combinations, this is quite successful. The second method is by a diazotisation and coupling aftertreatment. This involves diazotisation of a primary aromatic amino group in the disperse dye in the fibre and subsequent reaction of the diazonium ion with a suitable coupling component such as 3-hydroxy-2-naphthoic acid (BON acid after beta-oxy-naphthoic acid). Coupling in alkaline solution, as in the aftertreatment of direct dyes on cotton is less suitable for cellulose acetate because of the risk of surface hydrolysis of acetate groups. The amino disperse dye, for example CI Disperse Black is applied by conventional dyeing at 80 °C. After rinsing the orange fabric, the amino groups of the dye in the cellulose acetate are diazotised by reaction with a solution of sodium nitrite and hydrochloric acid at room temperature. After rinsing again, the fabric is treated with a dispersion of BON acid. This is prepared by precipitation of the free acid from a solution of its sodium salt in the presence of a dispersing agent. It is absorbed by the fibres at pH 4.5 exactly like a disperse dye. It reacts with the diazonium ion to form the dark navy pigment.
 |
| CI Disperse Black; dark navy pigment formed after the disperse dye is diazotised and then treated with a dispersion of BON acid |
Other sequences for dye application, diazotisation and coupling, are possible. The coupling component can be applied to the fibre after dyeing, as above, or even concurrently along with the amino disperse dye followed by its diazotisation. Once the colour has fully developed, scouring the material in soap or detergent solution at relatively low temperature removes pigment from the fibre surface and biproducts from the diazotisation and coupling sequence. If this is not done, inferior fastness properties result, particularly poor fastness to washing and rubbing.
Cellulose diacetate fabrics must be handled and dyed with care to avoid forming crease marks and stretching. Even at a dyeing temperature of 85 °C, the material is quite plastic and easily deformed. It is therefore preferable to dye such fabrics in open width using a jig machine. This is, however, not as simple as it might seem outlines some of the problems inherent in jig dyeing. With many disperse dyes, ending and listing effects are all too common, and are particularly noticeable when using less compatible combinations of dyes.
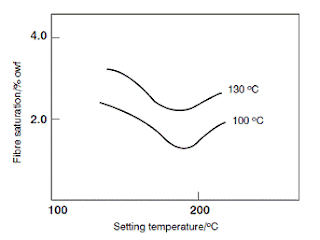
Cellulose triacetate is considerably more hydrophobic than diacetate and dyeing it with disperse dyes requires higher temperatures, but carries less risk of surface hydrolysis. The more compact internal structure gives lower dye diffusion rates in this fibre. It is normally dyed with disperse dyes at the boil. Dyeing temperatures up to 130 °C are possible and give improved washing and crocking fastness because of the better penetration of the dyes into the fibres. This is beneficial when dyeing heavy shades. It also allows use of dyes that are absorbed too slowly at 100 °C, thus increasing the range of available dyes. For dyeing deep shades, dyeing at the boil using a carrier such as diethyl phthalate is possible. This acts as a fibre swelling agent and thus accelerates dye absorption by increasing the diffusion rate. For a typical black, the amino disperse dye and coupling component are applied sequentially, or simultaneously. The black is developed by aftertreatment with a solution of sodium nitrite and hydrochloric acid that causes diazotisation of the dye and immediate coupling of the generated diazonium ion. Soaping removes surface colour, but usually a process called reduction clearing is preferred. In this, the dyed material is treated with a weakly alkaline solution of sodium hydrosulphite (hydros, Na2S2O4.2H2O), which reduces and eliminates the azo pigment on the fibre surface. Each combination of dye and coupling component requires its own particular dyeing and aftertreatment conditions so the dye supplier’s recommendations should be consulted. As for nylon, dry heat setting of cellulose triacetate fabrics improves their dimensional stability but reduces the dyeing rate. If heat setting or texturising has not been uniform, barré effects may be evident on fabrics made of filament yarns. Dyeing under pressure at above 100 °C increases the rate of dye migration and minimises barré effects. For heat pleating of cellulose triacetate materials after dyeing, it is essential to use disperse dyes that do not readily sublime from the heated fibre.